WHAT IS CORROSION?
Corrosion is the deterioration of a metal that results from a chemical reaction with its environment.


WHAT IS REINFORCEMENT?
They are bars, wires, strands, fibers, or other slender elements that are embedded in a matrix such that they act together to resist forces.

The corrosion of steel reinforcement in concrete is complex, but basically it is an electrochemical reaction similar to that of a simple battery. The composition of mild steel varies along its length and potential anodic (more negatively charged) and cathodic (positively charged) sites can be set up at various points.
The electrochemical potentials to form the corrosion cells may be generated in two ways:
- Composition cells may be formed when two dissimilar metals are embedded in concrete, such as steel rebars and aluminum conduit pipes, or when significant variations exist in surface characteristics of the steel.
- Concentration cells may be formed due to differences in concentration of dissolved ions near steel, such as alkalis, chlorides, and oxygen. The differences in electrochemical potential can arise from differences in the environment of the concrete. Electrochemical cells form also due to a variation in salt concentration in the pore water or due to a non- uniform access to oxygen. Thus, one of the two metals (or some parts of the metal when only one metal is present) becomes anodic and the other cathodic.
The fundamental chemical changes occurring at the anodic and cathodic areas are as follows;
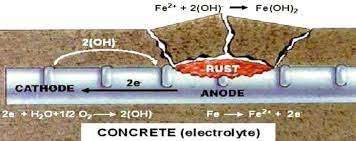
When there exists a difference in electrical potential along the steel in concrete, an electrochemical cell is set up: these forms anodic and cathodic regions, connected by the electrolyte in the form of the pore water in the hardened cement paste. The positively charged ferrous ions Fe++ at the anode pass into solution while the negatively charged free electrons e- pass through the steel into the cathode where they are absorbed by the constituents of the electrolyte and combine with water and oxygen to form hydroxyl ions (OH)-. These travels through the electrolyte and combine with the ferrous ions to form ferric hydroxide which is converted by further oxidation to rust.
FACTORS INFLUENCING CORROSION OF REINFORCEMENT
The factors which generally influence corrosion of reinforcement includes;
- pH value
- Moisture
- Oxygen
- Carbonation
- Chlorides
- Ambient temperature and relative humidity
- Severity of exposure
- Quality of construction materials
- Quality of concrete
- Cover to the reinforcement
DAMAGES TO CONCRETE DUE TO CORROSION OF STEEL REINFORCEMENT
- Formation of white patches.
- Brown patches along reinforcement.
- Occurrence of cracks.
- Formation of multiple cracks.
- Spalling of cover concrete.
- Snapping of bars.
- Buckling of bars and bulging of concrete.
In conclusion, corrosion of steel bars is highly detrimental to reinforced concrete and might undermine their elements serviceability and even cause structural failure. However, precautions can be taken to prevent the corrosion of steel bars. Such precautions are offered by the introduction of Armorsil West Africa range of admixtures that are sodium free which helps to protect reinforcement against corrosion.
Also, steel bars can be coated with our ARMORZINC PRIMER-20 against corrosion.





